
Saponification is a chemical reaction that occurs between oils/butters and lye water (or alkali). Glycerin (or glycerol) is a chemical byproduct that is also present in our end goods.
fats + lye water = soap + glycerin
Let's go over each of these substances together:
1. Oils/Butters
They are greasy or oily substances that aid in the utilization of vitamins and the maintenance of healthy skin in humans, plants, and animals; they are also the primary means by which the body stores energy. In plants, they typically accumulate in seeds (sunflower, sesame...) or fruit flesh (olive, avocado...).

Their chemical structure is made up of three branches attached to a skeleton (also known as triglycerides). This molecule is rather large and long, which is what gives the oil its relatively high viscosity. The harder the oil, the longer the arms.

I invite you to get information about oils on a more detailed level here (3min read). Spoiler alert: this is a bit technical, and you may recall some high school chemistry nightmares... However, understanding a topic correctly isn't always about swimming on the surface:) I guarantee you'll learn something interesting, not only about cosmetics but also about the food industry (trans-fats).
2. Lye water
When an alkali and water are combined, a high pH solution known as lye water is formed. It is very reactive and has enough strength to break down the fat molecules into soap (and glycerin). Lye is the only substance that can carry out this reaction. It is not possible to make soap without it.
The alkali used in soap production is either caustic soda (NaOH) or caustic potash (KOH):
NaOH is used to make a solid bar of soap
KOH is used to make liquid soap
a combination of the two will produce soap with a creamy texture, ideal for use as shaving cream.

3. Glycerin
The saponification reaction produces this substance as a byproduct. It has numerous proven skin benefits:
With its hydration/moisturization properties (emollient), it softens/soothes the skin.
antibacterial properties that are mild
protection from irritable stimuli
wound healing processes are accelerated
skin barrier function improvement
This substance is valuable and is sometimes removed from the soap so that it can be sold for a higher price than it would be worth in the soap itself. This is bad news for your skin, and the void left by the absence of glycerin is usually filled with cheaper chemical substitutes. Glycerin obtained naturally remains in natural soaps and accounts for approximately 8% of the final product. The rest is made up of soap molecules (>85%) and water. The water evaporates slowly, leaving the soaps.
4. How is soap cleaning?
Let's take again our representation of a soap molecule :
It has a long chain of carbons, which attracts fats/microbes/dirt,
the other end is negatively charged and attracts water molecules.

Dirt is unique in that it is insoluble in water. If a soap molecule comes into contact with dirt, fats, or microbes, the tail will be attracted and will "stab" the dirt.
The other part, attracted by water, wants to stay as far away from the dirt as possible but is connected to the tail. So you imagine an army of soap molecules stabbing the dirt from all directions until it forms a sphere, with the dirt in the center.

It's formed what's known as a micelle.
The dirt is now easily rinsed away because it is water soluble.
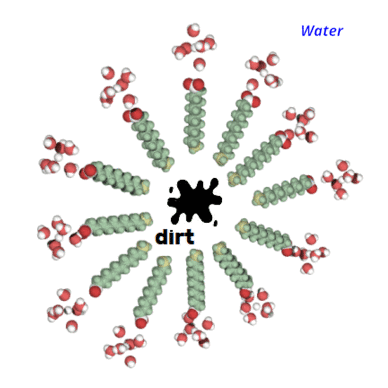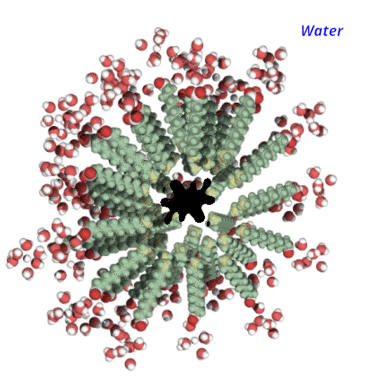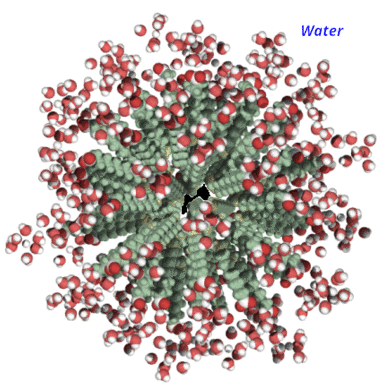
Because bacteria and viruses have lipid (fat) membranes, it has the same effect on them. When "stabbing" this barrier, it deconstructs it and exposes the microbe's interior to water, effectively killing it.
Corona update
"Anti-microbial soap" is a pleonasm, just as "vegan water" or "burning fire" are. All soaps are anti-microbial by nature. Don't always fall for stunning marketing claims. Which soap is best against viruses? The answer also well explained here.

Comments